Abstract
Introduction: ASXL1 mutations are frequently seen across the clinical spectrum of myeloid neoplasia. The most commonly identified ASXL1 mutation represents a single base duplication within an 8-guanine repeat at nucleotide position 1934 (c.1934dupG). Due to technical limitations of sequencing homopolymer regions, the ASXL1 c.1934dupG variant has been identified as potential artifact in some sequencing assays, though modern next generation sequencing assays and bioinformatics pipelines can generally accurately detect this mutation. However, a comprehensive comparison of ASXL1 c.1934dupG mutations versus non-c.1934dupG ASXL1 mutations have not been performed to date. Thus, we sought to explore a large dataset to determine if any biologic differences existed between these two groups.
Methods: Comprehensive genomic profiling by FoundationOne ®Heme testing was performed on patient samples with known or suspected myeloid neoplasms (MN). All MN patients ≥18 years old with 1 or more mutation were identified by internal database query. Patients were categorized as acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), non-chronic myeloid leukemia myeloproliferative neoplasms (MPN), or MDS/MPN overlap based on mutation and outside clinical and pathology data. Mutations with variant allele fractions (VAF) >1% were included for analysis, except for the ASXL1 c.1934dupG variant, which was only reported if the VAF was ≥15%. Fisher's exact tests were used to evaluate proportional differences between categorical variables, and Mann-Whitney U tests were used for comparisons of continuous variables.
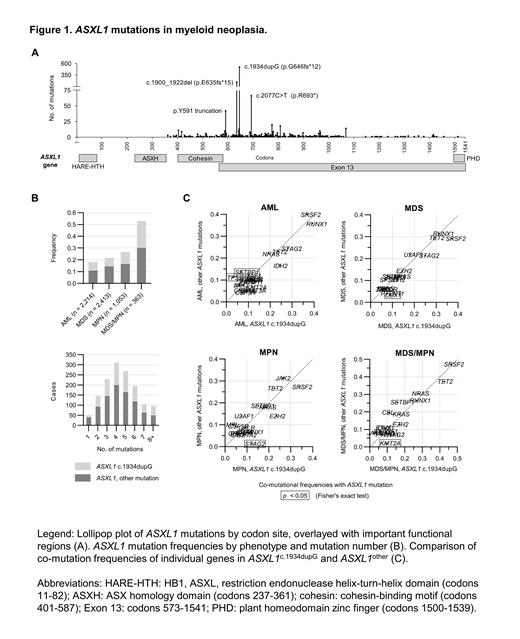
Results: Truncating ASXL1 mutations were identified in 1,414 included patients, occurring in 18% of AML and 26% of chronic myeloid neoplasms. Twenty-eight (2%) patients had multiple ASXL1 mutations, and ASXL1 was the sole mutated gene in 52 patients (4%). The most common ASXL1 mutation was c.1934dupG (Figure 1A), and this was the sole or dominant ASXL1 mutation in 520 cases (37%). The remaining 894 patients (63%) had one or more mutations at other sites in the ASXL1 gene (ASXL1other), with p.E635Rfs, p.R693*, and codon 591 mutations being the most common.
There were no significant differences in age, sex, or ancestry signatures between ASXL1c.1934dupG and ASXL1other. We noted slightly fewer ASXL1c.1934dupG mutations in patients with MDS (ASXL1c.1934dupG: ASXL1other 0.48:1) compared to AML (0.65:1, p = 0.03) and MPN (0.60:1, p = 0.01) and those in whom ASXL1 was the sole mutation (Figure 1B). However, these trends may have been due to VAF-based reporting thresholds, as ASXL1 VAFs were lower in singly mutated patients and those with an MDS diagnosis classification. Comparison of co-mutated genes with VAFs ≥15% between ASXL1c.1934dupG and ASXL1other revealed no significant difference in median non-ASXL1 mutations (each median 4, IQR 2-5, p = 0.74). When individual genes were assessed, co-mutation rates of STAG2 (p = 0.01) and KMT2A (p = 0.02) were higher in ASXL1c.1934dupG MNs, while SETBP1 (p = 0.01) mutations were more common with ASXL1other. In all MNs, the absolute differences in the frequency of mutations in ASXL1c.1934dupG versus ASXL1other were small. However, some differences emerged within phenotypic subgroups (Figure 1C). For instance, KMT2A rearrangements and STAG2 mutations were strongly associated with ASXL1c.1934dupG in MDS/MPN and MPN, with ASXL1c.1934dupG: ASXL1other ratios of 5:1 (p = 0.03) and 9:1 (p < 0.001), respectively. In contrast, AML patients with TP53 or SETBP1 mutations had a significantly higher mutation rate in ASXL1other (TP53: 11% vs. 3% in ASXL1c.1934dupG, p < 0.01; SETBP1: 14% vs. 7%, p=0.04). We further identified that other specific ASXL1 mutations were more commonly co-mutated in AML with TP53 (ASXL1 p.R693*, p < 0.001) or SETBP1 (ASXL1 p.R404*, p < 0.001).
Conclusion: Our results confirm the ASXL1 c.1934dupG variant occurs in a similar patient population to other ASXL1 mutations, and further supports its pathogenicity in myeloid neoplasia. Subset analysis suggests that ASXL1c.1934dupG and ASXL1other may be associated with certain phenotypic and co-mutational tendencies. Thus, ASXL1 mutation site may be an important variable in some patients and should be considered in future mechanistic and clinical studies. Further study is warranted to determine whether clinical outcomes are affected by different ASXL1 mutations.
Haberberger: Foundation Medicine, Inc.: Current Employment. Ferguson: Foundation Medicine Inc: Current Employment, Other: ownership.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal